CHEM134 Week 7 Lab 7 Assignment
- $20.00
- Question: Please upload (in PDF format) a picture of your experimental setup and data collection process (step 21 in the lab document). To receive credit, the picture must be clear, you must be clearly visible in the picture, your setup must be clearly visible, and a label must be clearly visible containing your name, the lab title, and the date. This must be a single PDF file in order to receive credit.
- Question: Please upload (in PDF format) your completed Table 1 (step 32 in the lab document). This must be a single PDF file in order to receive credit. NOTE: If your document is not clear, organized, and all categories properly labeled/titled, or any part of it is unclear to your instructor, you will not receive credit.
- Question: Please upload (in PDF format) your pH vs. NaOH (mL) derivative graph as instructed in the lab procedure (Step 29). Be sure the graph is clear and properly labeled (axes, name, Student ID, lab title, date). NOTE: If your graph is not properly labeled or is not clear, or if any part of it cannot be read by your instructor, you will not receive credit.
- Question: Please upload (in PDF format) your pH vs. NaOH (mL) titration curve graph as instructed in the lab procedure (Step 30). Be sure the graph is clear and properly labeled (axes, name, Student ID, lab title, date). NOTE: If your graph is not properly labeled or is not clear, or if any part of it cannot be read by your instructor, you will not receive credit.
- Question: Please upload (in text format) your Vernier equilibrium constant data run table as instructed in the lab procedure (Step 24). Be sure the data is clearly labeled, including your name, ID, lab title, and date, as described in the instructions. All three data columns should be present. NOTE: If your table is not properly labeled or is not clear, or if any part of it cannot be read by your instructor, you will not receive credit.
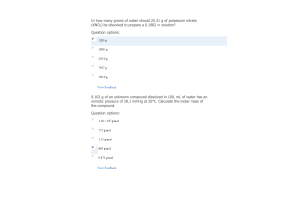
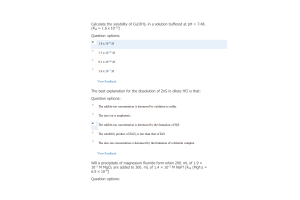
- Question: (a) According to your experimental data, what volume of 0.10 M NaOH represents the half- equivalence (a.k.a. half-neutralization) point in this titration? (b) What is the pH of the solution at the half-neutralization point? (c) Using this info, what is the experimental pKa for acetic acid in this reaction? Explain your answer. (d) Using the pKa value above, what is your experimental Ka for acetic acid? (You must show all work to receive credit)
- Question: Look up the accepted “actual” Ka value for acetic acid. How does your value compare? Calculate the percent error for your experimental value. (You must show all work to receive credit.)
- Question: What are some possible sources of error in your experiment, and how could you account for these sources in future experimental runs? Discuss in detail.













-300x200.png)