CHEM134 Week 16 Lesson 8 QuizScore 100%
- $25.00
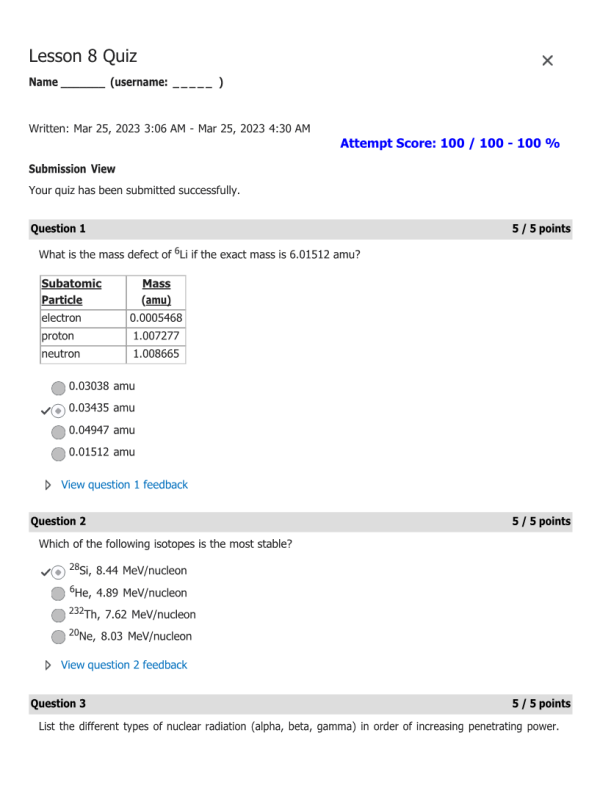
- Question: What is the mass defect of 6Li if the exact mass is 6.01512 amu?
- Question: Which of the following isotopes is the most stable?
- Question: List the different types of nuclear radiation (alpha, beta, gamma) in order of increasing penetrating power.
- Question: If the mass of one neutron is 1.00866 amu, the mass of one proton is 1.00728 amu, and the mass of 14C nucleus is 13.99995 amu, calculate the binding energy for the 14C nucleus.
- Question: What is the mass change in grams for the reaction 2 NO2(g) → N2O4(g)? The enthalpy for the reaction is -57.2 k
- Question: Elements with _______ atomic mass are best possible candidates for a fission reaction.
- Question: If a log contains 60.0% of the 14C present in a living tree, how long has the log been dead? The half-life of 14C is 5730 years.
- Question: If the age of the Earth is 4.5 billion years and the half-life of 40K is 1.26 billion years, what percent of the Earth's original amount of 40K remains today?
- Question: Alpha particles are identical to:
- Question: Which of the following is characteristic of a nuclear reaction? I. Electrons in atomic orbitals are involved in the breaking and forming of bonds II. Elements are converted from one to another III. Reaction rates are typically not affected by catalysts IV. Atoms are rearranged by the breaking and forming of chemical bonds
- Question: Consider a certain type of nucleus that has a half-life of 32 min. Calculate the percent of original sample of nuclides remaining after 2.1 hours have passed.
- Question: Elements with _______ atomic mass are best possible candidates for a fusion reaction.
- Question: An unstable isotope of rhenium, 191Re, has a half-life of 9.8 minutes and is a beta producer. What is the other product of the reaction?
- Question: Carbon dating may be used to date (once living) materials that are between 100 and 40,000 years old. The half-life of the first-order decay of carbon-14 is 5730 years. What percentage of carbon-14 remains in a sample after 1.99 x 104 years?
- Question: The number of a certain radioactive nuclide present in a sample decays from 160. to 20. in 39 minutes. What is the half-life of this radioactive species?
- Question: The rate constant for the beta decay of thorium-234 is 2.881 x 10–2 day–1. What is the half-life of this nuclide?
- Question: Consider a certain type of nucleus that has a rate constant of 2.03 x 10–2 min–1. Calculate the time required for the sample to decay to one-fourth of its initial value.
- Question: Calculate the mass defect for the formation of phosphorus-31. The mass of a phosphorus-31 nucleus is 30.973765 amu. The masses of a proton and a neutron are 1.00728 and 1.00866 amu, respectively.
- Question: Beta particles are identical to:
- Question: Decay of ruthenium-93 by positron emission yields













-300x200.png)