CHEM134 Week 2 Lesson 1 Quiz
- $25.00
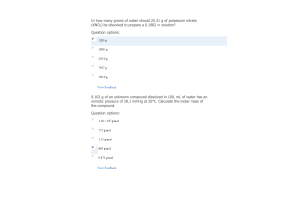
- Question: A KCl solution is prepared by dissolving 40.0 g KCl in 250.0 g of water at 25°C. What is the vapor pressure of the solution if the vapor pressure of water at 25°C is 23.76 mm Hg?
- Question: Calculate the molality of a 20.0% by mass ammonium sulfate (NH4)2SO4 solution. The density of the solution is 1.117 g/mL.
- Question: A solution is prepared by dissolving 17.75 g sulfuric acid, H2SO4, in enough water to make 100.0 mL of solution. If the density of the solution is 1.1094 g/mL, what is the molality?
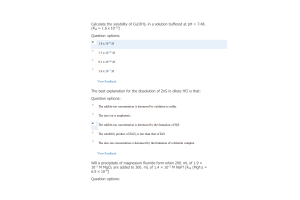
- Question: The Henry's law constant for O2 in water at 25 °C is 1.3 x 10-3 mol/kg·bar. What partial pressure of O2 (in atm) is necessary to achieve an equilibrium concentration of 2.62 x 10-3 mol/kg O2? (1 atm = 0.9869 bar)
- Question: What is the equilibrium partial pressure of water vapor above a mixture of 52.1 g H2O and 37.1 g HOCH2CH2OH at 55 °C. The partial pressure of pure water at 55.0 °C is 118.0 mm Hg. Assume ideal behavior for the solution.
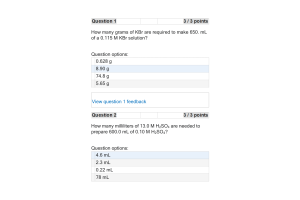
- Question: How many milliliters of 13.0 M H2SO4 are needed to prepare 600.0 mL of 0.10 M H2SO4?
- Question: Which of the following statements is INCORRECT?
- Question: Which of the following statements concerning osmosis is/are CORRECT?
- Question: A solution has a density of 1.023 g/mL and a concentration of 0.0800 g/dL. What is the concentration in parts per million?
- Question: Assuming ideal behavior, which of the following aqueous solutions should have the highest boiling point (kb = 0.5121 °C/m)?
- Question: Which response lists all the following pairs that are miscible liquids:
- Question: In how many grams of water should 25.31 g of potassium nitrate (KNO3) be dissolved to prepare a 0.1982 m solution?
- Question: What is the molar mass of a nonpolar molecular compound if 6.34 g dissolved in 53.4 g benzene begins to freeze at 2.81 °C? The freezing point of pure benzene is 5.50 °C and the freezing point depression constant, Kf, is -5.12 °C/m.
- Question: Which of the following statements concerning solubility is/are CORRECT?
- Question: The rubbing alcohol sold in drug stores often is composed of 70% isopropyl alcohol and 30% water. In this solution:
- Question: How many molecules of sucrose (table sugar), C12H22O11, dissolved in 450.0 g of water are needed to make a 1.30 m solution?
- Question: What is the mole fraction of ethanol in a solution made by dissolving 14.6 g of ethanol, C2H5OH, in 53.6 g of water?
- Question: Determine the mass percent HCl in a 1.2 M solution of hydrochloric acid with a density of 1.019 g/mL?
- Question: How many grams of KBr are required to make 650. mL of a 0.115 M KBr solution?
- Question: A KCl solution is prepared by dissolving 40.0 g KCl in 250.0 g of water at 25°C. What is the vapor pressure of the solution if the vapor pressure of water at 25°C is 23.76 mmHg?













-300x200.png)