CHEM134 Week 8 Lesson 4 QuizScore 100%
- $25.00
- Question: Which of the following substances is never a Brønsted-Lowry acid in an aqueous solution?
- Question: Which of the following is the most acidic solution?
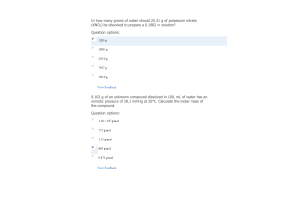
- Question: In a solution prepared by dissolving 0.100 mole of propanoic acid in enough water to make 1.00 L of solution, the pH is observed to be 2.945. The Ka for propanoic acid (HC3H5O2) is:
- Question: Molecules or ions that can alternately behave as either a Brønsted-Lowry acid or base are called:
- Question: What is the strongest monoprotic acid of the following set if all the acids are at 0.100 M concentration?
- Question: 24.00 mL of a 0.25 M NaOH solution is titrated with 0.10M HCl. What is the pH of the solution after 24.00 mL of the HCl has been added?
- Question: What is the [CH3CO2-]/[CH3CO2H] ratio necessary to make a buffer solution with a pH of 4.44? Ka = 1.8 × 10-5 for CH3CO2H.
- Question: Calculate the pOH of a 4.6 M solution of HCl.
- Question: What is the pH of 0.109 M HCl(aq) at 25 °C?
- Question: A 50.0 mL sample of 0.10 M HNO2 (Ka = 4.0 x 10–4) is titrated with 0.12 M NaOH. The pH after 25.0 mL of NaOH have been added is
- Question: What is the magnitude of the change in pH when 0.005 moles of HCl is added to 0.100 L of a buffer solution that is 0.100 M in CH3CO2H and 0.100 M NaCH3CO2? The Ka for acetic acid is 1.8 × 10-5.
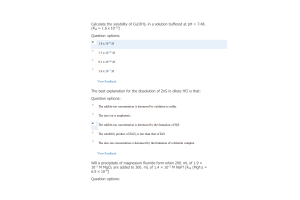
- Question: When 0.125 mol HF is dissolved in water at 292 K, and diluted to a volume of 1.0 L, 7.23% of the HF dissociates to form F –(aq). What is the equilibrium constant for the reaction?
- Question: What is the pH of 0.492 M aqueous ethylamine? (Kb of C2H5NH2 = 4.3E-4)
- Question: What is the concentration of H+ in a 2.5 M HCl solution?
- Question: Which one of these salts will form a basic solution on dissolving in water?
- Question: Which of the following solutions will be the best buffer at a pH of 9.26? (Ka for HC2H3O2 is 1.8 x 10–5, Kb for NH3 is 1.8 x 10–5).
- Question: What is the molarity of a sodium hydroxide solution if 27.9 mL of this solution reacts exactly with 22.30 mL of 0.253 M sulfuric acid?
- Question: Which is a net ionic equation for the neutralization reaction of a weak acid with a weak base?
- Question: 40.0 ml of an acetic acid of unknown concentration is titrated with 0.100 M NaOH. After 20.0 mL of the base solution has been added, the pH in the titration flask is 5.10. What was the concentration of the original acetic acid solution?
- Question: Which of the following does not fit the definition of a Brønsted Base?













-300x200.png)