CHEM134 Week 8 Midterm
- $35.00
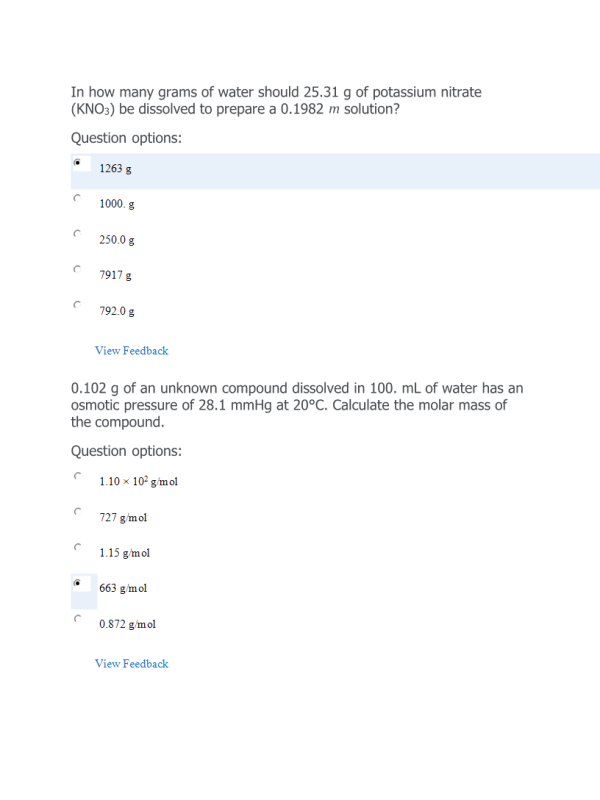
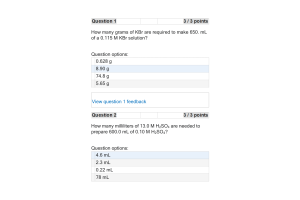
- Question: In how many grams of water should 25.31 g of potassium nitrate (KNO3) be dissolved to prepare a 0.1982 m solution?
- Question: 0.102 g of an unknown compound dissolved in 100. mL of water has an osmotic pressure of 28.1 mmHg at 20°C. Calculate the molar mass of the compound.
- Question: Calculate the molality of a 20.0% by mass ammonium sulfate (NH4)2SO4 solution. The density of the solution is 1.117 g/mL.
- Question: The osmotic pressure of blood is 7.65 atm at 37 °C. What mass of glucose (C6H12O6, molar mass = 180.2 g/mol) is needed to prepare 2.66 L of solution for intravenous injection? The osmotic pressure of the glucose solution must equal the osmotic pressure of blood.
- Question: Which of the following statements concerning osmosis is/are CORRECT?
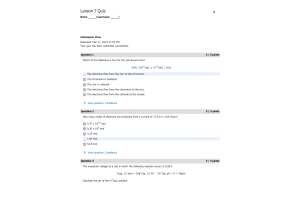
- Question: The decomposition of dinitrogen pentoxide is described by the chemical equation 2 N2O5(g) → 4 NO2(g) + O2(g) If the rate of appearance of O2 is equal to 2.40 mol/min at a particular moment, what is the rate of disappearance of N2O5 at that moment?
- Question: Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2ClO2(aq) + 2OH–(aq) → ClO2–(aq) + ClO3–(aq) + H2O(l) Under a certain set of conditions, the initial rate of disappearance of chlorine dioxide was determined to be 2.30 x 10–1 M/s. What is the initial rate of appearance of chlorite ion under those same conditions?
- Question: Concerning the rate law, Rate = k[A]2[B], what are appropriate units for the rate constant k?
- Question: At 700 K, the rate constant for the isomerization reaction from cyclopropane to propene is 6.2 × 10–4 min–1. If 20% of a sample of cyclopropane is isomerized to propene, how many minutes did it take?
- Question: Cerium(IV) ion reacts with thallium(I) ion in a one-step reaction shown below: 2 Ce4+(aq) + Tl+(aq) → 2 Ce3+(aq) + Tl3+(aq). If the rate law is: Rate = k[Ce4+]2[Tl+], what is the overall order of the reaction?
- Question: Sulfuryl chloride decomposes to sulfur dioxide and chlorine by the following reaction: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) Kc = 0.045 at 648 K If an initial concentration of 6.76 x 10-2 M SO2Cl2 is allowed to equilibrate, what is the equilibrium concentration of Cl2?
- Question: Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) ⇌ 2Cu2O(s), K14CuO(s) ⇌ 2Cu2O(s) + O2(g), K2 what is K for the system 2Cu(s) + O2(g) ⇌ 2CuO(s) equivalent to?
- Question: Assume that the following chemical reaction is at equilibrium. 2 ICl(g) ⇌ I2(g) + Cl2(g) ΔH° = +26.9 kJ At 25 °C, Kp = 2.0 x 105. If the temperature is increased to 45 °C, which statement applies?
- Question: What is the value of n for the reaction below? NH4NO3(s) ⇌ N2O(g) + 2 H2O(g)
- Question: A catalyst increases the overall rate of reaction by lowering the activation energy, Ea, for
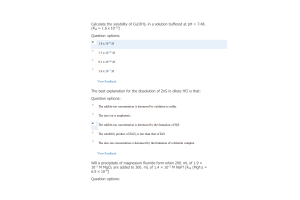
- Question: The pH of aqueous 0.35 M hypobromous acid, HBrO, is 4.53. What is the Ka of this acid?
- Question: Aniline, (C6H5NH2, Kb = 4.3 × 10-10 at 25°C) is an industrially important amine used in the making of dyes. Determine the pH of an aniline solution made by dissolving 3.90 g of aniline in enough water to make 100 mL of solution.
- Question: A 0.14 M HNO2 solution is 5.7% ionized. Calculate the H+ ion concentration.
- Question: Which of the following species is the strongest acid in an aqueous solution?
- Question: Calculate the [H+] in a solution that is 0.17 M in NaF and 0.25 M in HF. (Ka = 7.2 x 10–4)
- Question: Calculate vapor pressure at 60°C for a solution prepared by dissolving 14.0 mol naphthalene in 86.0 mol ethanol?
- Question: What is the osmotic pressure in atm produced by a 1.02 M lactose (C12H22O11) solution at 25°C?
- Question: The rate constant for a particular reaction is 7.2 x 10-3 s-1 at 35 °C and 1.7 x 10-2 s-1 at 80 °C. What is the activation energy for the reaction?
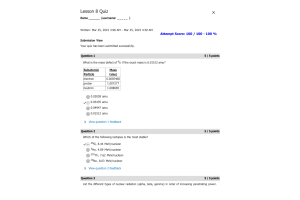
- Question: For the following process:
- Question: Write the equilibrium constant for the following reaction: CaO(s) + CO2(g) → CaCO3(s) The equilibrium concentration of a reaction is generally represented as
- Question: If HCl(g) initially at a partial pressure of 0.445 atm; is reacting with I2(s): 2 HCl(g) + I2 (s) ⇌ 2 HI (g) + Cl2 (g) Keq = 3.9 x 10-33 at 25 oC Calculate the final partial pressures at equilibrium.
- Question: 50.0 mL of 0.50 M NaOH is added to a 250 mL buffer solution containing 0.30 M NH3 and 0.36 M NH4Cl. What is the pH of the solution after the addition of the base?
- Question: A solution of sodium acetate (CH3COONa) at 25 °C has a pH of 7.5. What is the concentration of CH3COONa in the solution? (Ka for CH3COOH is 1.8 × 10−5) sodium acetate is a salt of week acid and strong base













-300x200.png)