CHEM134 Week 14 Lesson 7 Quiz Score 95%
- $25.00
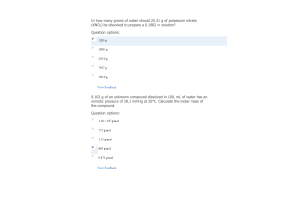
- Question: Which of the following is true for the cell shown here?
- Question: How many moles of electrons are produced from a current of 17.6 A in 3.00 hours?
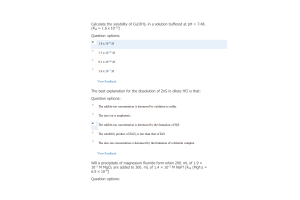
- Question: The measured voltage of a cell in which the following reaction occurs is 0.96 V H2(g, 1.0 atm) + 2Ag+(aq, 1.0 M) → 2H+(aq, pH = ?) + 2Ag(s) 5 / 5 points Calculate the pH of the H+(aq) solution.
- Question: The following reaction occurs in basic solution: F2 + H2O → O2 + F– hen the equation is balanced, the sum of the coefficients is:
- Question: Given the following notation for an electrochemical cell Pt(s) | H2(g) | H+(aq) || Ag+(aq) | Ag(s) what is the balanced overall (net) cell reaction?
- Question: Given the following reaction in acidic media: Fe2+ + Cr2O72– → Fe3+ + Cr3+ answer the following question: The coefficient for water in the balanced reaction is:
- Question: Which one of the following reactions will occur spontaneously at standard-state conditions and 25°C?
- Question: What species is oxidized in the following reaction? CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s)
- Question: Complete and balance the following redox equation. What is the coefficient of when the equation is balanced with the set of smallest whole-number coefficients?
- Question: A galvanic cell employs the reaction Mg2+(aq) + Cu(s) → Mg(s) + Cu2+(aq) and NaNO3 is the salt used in the salt bridge. During the course of the reaction.
- Question: Consider the following standard reduction potentials in acid solution:
- Question: During an electrochemical reaction, electrons move through the external circuit toward the_________and positive ions in the cell move toward the ______ _.
- Question: For a galvanic cell, the cathode has a ________ sign and is the site of _______ _.
- Question: How many electrons are transferred in the following reaction?
- Question: Which of the following terms can be used to describe an electrochemical cell in which a spontaneous chemical reaction generates an electric current?
- Question: Predict the products of the electrolysis of aqueous potassium chloride KCl(aq).
- Question: What quantity of charge is required to reduce 21.4 g of CrCl3 to chromium metal? (1 Faraday = 96,485 coulombs).
- Question: A salt bridge is used to:
- Question: What is the oxidation state of Cr in Cr2O72–?
- Question: Calculate ΔG° for the electrochemical cell














-300x200.png)