CHEM134 Week 6 Lesson 3 Quiz
- $25.00
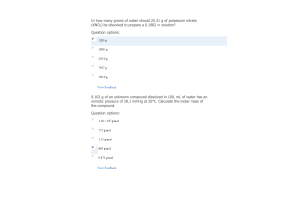
- Question: Given the equilibrium constants for the following reactions:
- Question: If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g) ⇌ 2NO(g) + O2(g) reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
- Question: Which is the correct equilibrium constant expression for the following reaction?
- Question: A mixture of carbon monoxide, hydrogen, and methanol is at equilibrium. The balanced chemical equation is: CO(g) + 2H2(g) ⇌ CH3OH(g) At 250°C, the mixture contains 0.0960 M CO, 0.191 M H2, and 0.150 M CH3OH. What is the value for Kc?
- Question: The equilibrium constant expression for the reaction: 2BrF5 (g) ⇌ Br2 (g) + 5F2 (g)
- Question: The decomposition of nitrosyl bromide is exothermic: 2 NOBr(g) ⇌ 2 NO(g) + Br2(g) Which of the following changes in reaction condition will shift the reaction to the left?
- Question: A catalyst increases the overall rate of reaction by lowering the activation energy, Ea, for
- Question: For the reaction at equilibrium 2SO3 ⇌ 2SO2 + O2 ΔHºrxn= 198 kJ/mol increasing the reaction temperature would:
- Question: Sulfuryl chloride decomposes to sulfur dioxide and chlorine by the following reaction: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) Kc = 0.045 at 648 K If an initial concentration of 6.76 x 10-2 M SO2Cl2 is allowed to equilibrate, what is the equilibrium concentration of Cl2?
- Question: Write a balanced chemical equation which corresponds to the following equilibrium constant expression: Keq = [Pb2+] [F–]2
- Question: Assume that the following chemical reaction is at equilibrium. 2 ICl(g) ⇌ I2(g) + Cl2(g) ΔH° = +26.9 kJ At 25 °C, Kp = 2.0 x 105. If the temperature is increased to 45 °C, which statement applies?
- Question: What is the value of n for the reaction below? NH4NO3(s) ⇌ N2O(g) + 2 H2O(g) Kp is related to Kc by the equation Kp = Kc(RT)Δn.
- Question: Which one of the following statements about the equilibrium constant, Kp, is false?
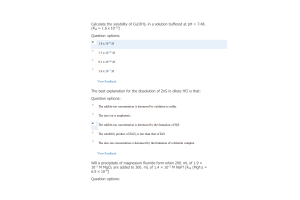
- Question: Ozone is formed from oxygen by the following reaction: 3/2 O2(g) ⇌ O3(g) Kp = 3.4 x 10-38 at 220 K. Calculate the value of Kc.
- Question: Consider the following reaction: 2HF(g) ⇌ H2(g) + F2(g) K = 1.00 10–2 Given 1.00 mol of HF(g), 0.386 mole of H2(g), and 0.750 mole of F2(g) are mixed in a 5.00 L flask, determine the reaction quotient, Q.
- Question: What will be the total pressure in the following system at equilibrium: N2O4(g) ⇌2NO2(g) K = 4.66 x 10–8 at -80°C if we introduce 0.047 mol of N2O4 into a 1.0 L vessel at -80°C and let equilibrium become established?
- Question: If the reaction quotient, Q, is greater than K in a gas phase reaction, then:
- Question: For the reaction given below, 2.00 moles of A and 3.00 moles of B are placed in a 6.00 L container. A(g) + B(g) ⇌ C(g) At equilibrium, the concentration of A is 0.282 mol/L. What is the concentration of B at equilibrium?
- Question: If 0.617 mol PCl5 is placed in a 1.81 L flask and allowed to reach equilibrium at a given temperature, what is the final concentration of Cl2 in the flask? PCl5(g) ⇌ PCl3(aq) + Cl2(g) Kc = 0.47
- Question: Which one of the following statements does not describe the equilibrium state?













-300x200.png)